Ficlatuzumab (anti-HGF IgG1 mAb)
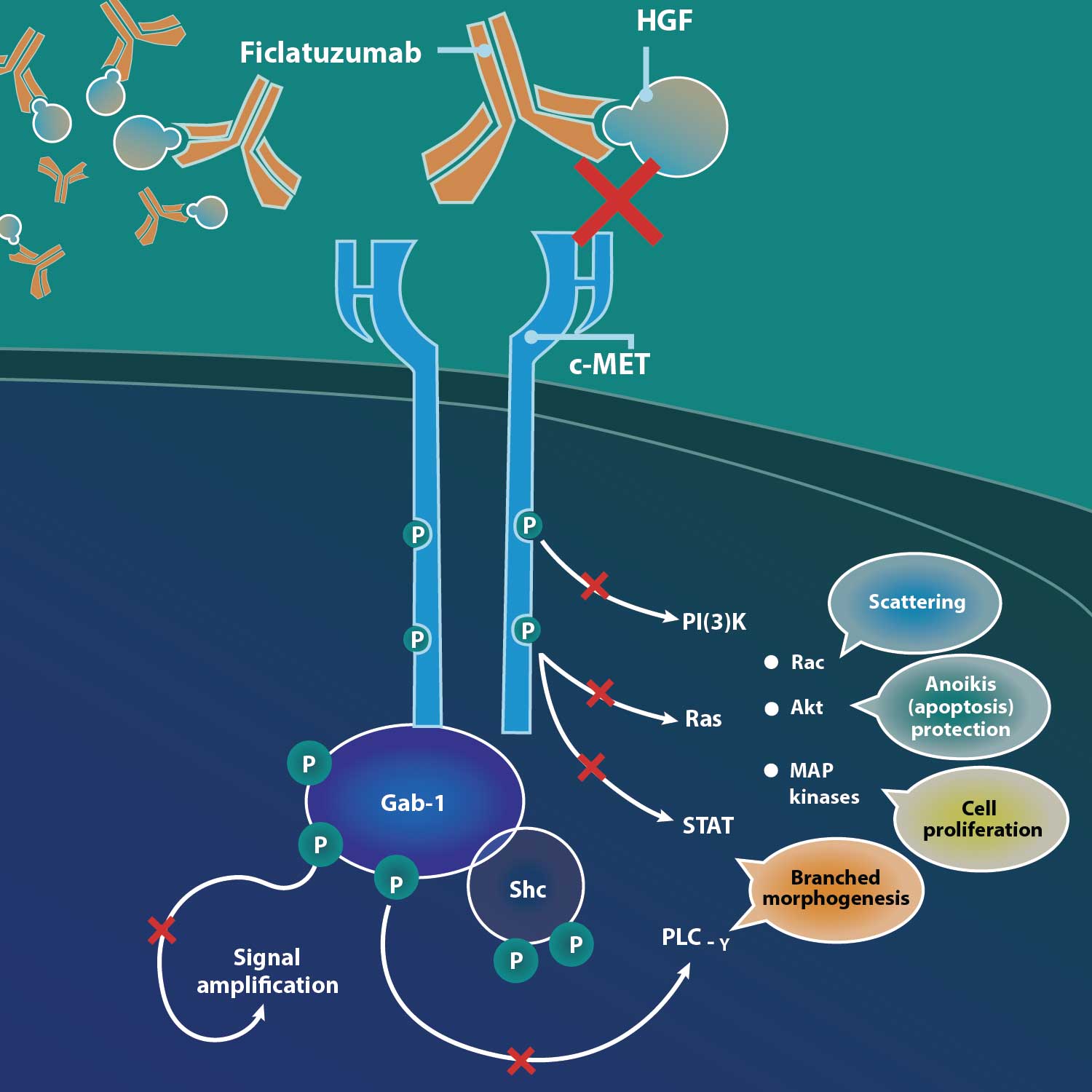
Ficlatuzumab (also known by its research code AV-299) is a potent humanized IgG1 monoclonal antibody that targets hepatocyte growth factor, or HGF. The HGF/cMET pathway is implicated as an escape mechanism for epidermal growth factor receptor, or EGFR, blockade. By binding to the HGF ligand with affinity and specificity, ficlatuzumab has demonstrated differentiated inhibition of HGF/cMET downstream signaling and demonstrated a strong additive anti-tumor effect in preclinical studies and early clinical trials.
Ficlatuzumab in HNSCC
In 2022, the U.S. FDA designated the investigation of AV-299 (ficlatuzumab) and Erbitux® (cetuximab) for relapsed/recurrent (R/R) head and neck squamous cell carcinoma (HNSCC) as a Fast Track development program. A drug that receives Fast Track designation is eligible for more frequent meetings and written communication with FDA, eligibility for accelerated approval and priority review (if criteria are met), and rolling review of a future marketing application.
AVEO has reported results from a randomized phase 2 clinical trial of ficlatuzumab with or without cetuximab (ERBITUX®), in patients with (pan-refractory) recurrent or metastatic (R/M) HNSCC, including data related to progression-free survival (PFS) and anti-tumor activity in patients who were HPV-negative randomized to the combination arm. (See study results, “Randomized Phase II Trial of Ficlatuzumab With or Without Cetuximab in Pan-Refractory, Recurrent/Metastatic Head and Neck Cancer”)
AVEO has initiated a multicenter, randomized, double blind, placebo-controlled, phase 3 clinical trial of ficlatuzumab in combination with cetuximab in participants with recurrent or metastatic (R/M) HPV-negative head and neck squamous cell carcinoma (FIERCE-HN). AVEO is currently enrolling patients in this study. (For more information go to FIERCEHN.com or clinicaltrials.gov)
Ficlatuzumab in other indications
Ficlatuzumab has also been studied in combination with chemotherapy in pancreatic cancer and acute myeloid leukemia in early stage clinical trials. Both studies demonstrated promising efficacy activity and an acceptable tolerability profile. AVEO continues to evaluate additional opportunities for further clinical development of ficlatuzumab in these therapeutic areas.
HGF/cMET as a Therapeutic Target
HGF is the sole ligand for the c-MET receptor and this pathway is frequently dysregulated in a broad range of human cancers. HGF binding with c-MET can activate multiple intracellular signaling pathways and lead to both tumor growth and metastatic progression of cancer cells. HGF-induced MET activation is also an escape mechanism for EGFR inhibition, leading to resistance. Ficlatuzumab has demonstrated differentiated inhibition of HGF.
- High affinity (pM) and slow off-rate for HGF
- High potency (nM) inhibiting all biological activities of HGF, including autocrine/paracrine activation loops
Ficlatuzumab is an investigational drug and has not yet been approved by the FDA.